Articles
Flávia Helena Pereira 1
Danilo Donizetti Trevisan 2
Daniela Santos Lourenço 3
Juliana Bastoni da Silva 4
Maria Helena de Melo Lima 5
1 ![]() 0000-0001-9331-7020. Instituto
Federal de Educação, Ciência e Tecnologia do Sul de Minas Gerais, Brazil. flavia.pereira@ifsuldeminas.edu.br
0000-0001-9331-7020. Instituto
Federal de Educação, Ciência e Tecnologia do Sul de Minas Gerais, Brazil. flavia.pereira@ifsuldeminas.edu.br
2 ![]() 0000-0002-6998-9166. Universidade
Federal de São João del-Rei, Brazil. ddtrevisan@ufsj.edu.br
0000-0002-6998-9166. Universidade
Federal de São João del-Rei, Brazil. ddtrevisan@ufsj.edu.br
3 ![]() 0000-0002-4176-300X. Universidade Federal de Alfenas, Brazil. daniela.maximo@unifal.edu.br
0000-0002-4176-300X. Universidade Federal de Alfenas, Brazil. daniela.maximo@unifal.edu.br
4 ![]() 0000-0002-6642-8910. Universidade
Federal do Tocantins, Brazil. juliana.bastoni@uft.edu.br
0000-0002-6642-8910. Universidade
Federal do Tocantins, Brazil. juliana.bastoni@uft.edu.br
5 ![]() 0000-0001-6521-8324. Universidade Estadual de
Campinas, Brazil. melolima@unicamp.br
0000-0001-6521-8324. Universidade Estadual de
Campinas, Brazil. melolima@unicamp.br
Received: 10/01/2019
Sent to peers: 06/03/2019
Approved by peers: 24/05/2019
Accepted: 12/06/2019
10.5294/aqui.2019.19.3.2
Theme: Chronic care.
Contribution to the discipline: The findings of this study reinforce the importance of health professionals integrally knowing the patient with diabetes mellitus type 2 (DM2), making the approach and evaluation of the sleep pattern part of the clinical practice, so that sleep hygiene interventions are proposed according to each need. In addition, sleep hygiene actions can improve disease-related stress and quality of life, which reflects on positive attitudes to control the disease.
Para citar este artigo / Para citar este artículo / To reference this article: Pereira FH, Trevisan DD, Santos D, da Silva JB, Lima MHdM . Effect of educational strategies on the sleep quality of people with diabetes: randomized clinical trial. Aquichan 2019; 19(3): e1932. DOI: https://doi.org/10.5294/aqui.2019.19.3.2
|
ABSTRACT Objective: To evaluate the effect of educational
strategies on sleep quality and its relation to diabetes-related distress and
glycemic control in people with type 2 diabetes mellitus (DM2). Keywords (source DeCS): Sleep; Sleep Hygiene; Diabetes Mellitus, Type 2; Stress, Psychological; Nursing. |
Resumen Objetivo: evaluar el efecto de estrategias
educativas en la calidad del sueño y su relación con el estrés emocional
asociado con diabetes en personas con DM2. Palabras clave (fuente DeCS): Sueño; higiene del sueño; Diabetes Mellitus tipo 2; estrés psicológico; Enfermería. |
Resumo Objetivo: avaliar o efeito de estratégias educativas
sobre a qualidade do sono e sua relação com o estresse emocional associado com o diabetes em pessoas com DM2. Palavras-chave (fonte DeCS): Sono; higiene do sono; Diabetes Mellitus tipo 2; estresse psicológico; Enfermagem. |
Introduction
The International Diabetes Federation (IDF) estimated in 2017 that approximately 425-million people live with Diabetes Mellitus (DM) in the world. In 2045, this number could exceed 629-million people (1). In that same year, approximately 12.4-million Brazilian adults between 20 and 79 years of age lived with DM, which led the country to be considered the first in South and Central America, and the fourth largest in the world with adults with DM, considering the total population (1). Diabetes Mellitus type 2 (DM2) is the most prevalent type of this chronic condition.
In the literature, the relationship between DM2 and sleep disorders is well established (2-4). People with this chronic condition commonly have alterations in sleep pattern, like difficulty in initiating and maintaining sleep, daytime sleepiness, and poor sleep quality (5-8). The study showed that 55 % of the people with DM2 had low quality of sleep, according to the Pittsburgh Sleep Quality Inventory (PSQI) (9). The low quality of sleep can affect the physical and emotional wellbeing, and contribute negatively in the treatment of DM2 (9).
In addition, because of the complex condition that DM2 may require, many patients might experience difficulties in modifying their lifestyles, which involves changes in eating habits, performance of physical activity, and adherence to drug treatment; thus, they can suffer important psychological impacts, such as increased levels of diabetes-related emotional distress. High levels of diabetes-related emotional distress are significantly related to poor sleep quality and higher levels of glycated hemoglobin (A1c) in different scenarios and types of studies (9-11).
Some non-pharmacological measures, such as interventions involving cognitive-behavioral therapy, exposure to light, performance of physical activity, sleep hygiene, and relaxation therapies are aimed at improving the quality of sleep and can contribute to diminishing diabetes-related distress (12-16); consequently, an increase in self-care may be observed to manage DM. However, the relationship between sleep quality and diabetes-related distress still remains close, suggesting the need for further studies to better elucidate it. In this sense, the aim of this study was to evaluate the effect of educational strategies based on sleep hygiene on the quality of sleep and its relation with diabetes-related emotional distress in people with DM2.
Method
Study design
This was a randomized clinical trial, parallel and composed of two arms — Group 1 (G1) and Group 2 (G2). The study took place in four units of Family Health Strategies (FHS) from a municipality of the south of Minas Gerais, Brazil. The study was conducted between September 2015 and August 2016, approved by the Ethics Committee in local research, with favorable concept (1.183.930/2016), and registered in the Brazilian Registry of Clinical Trials (ReBEC, RBR-468WM2). The Consolidated Statement of Reporting Trials (CONSORT) (17) was followed for randomization, blinding, monitoring, and analyzing the data.
Participants
Participants in this study were people with DM2, who are adults and who treat this chronic condition in the FHS units where this study was carried out. The study included users who had been on DM2 treatment for at least six months, who had poor sleep quality (score > 5 points), measured through the PSQI (18), who were not on any pharmacological or non-pharmacological treatment that interfered with sleep and who had telephone access (mobile or landline). The study excluded participants who had insufficient cognitive behavior, according to the educational levels, through the Mini-mental State Examination (MMSE) instrument (19), and those who worked nights. The discontinuity criteria were: No attendance or withdrawal at any of the scheduled meetings and start of pharmacological/non-pharmacological treatment that interfered with sleep.
Sample size
The sample size was estimated by considering the methodology to calculate the sample according to the unpaired student t test from the results observed in a prior study (20). A power of 80 % and a level of significance of 1.25 % were assumed in the sample calculations. This level of significance was determined through the Bonferroni correction. After considering a 20 % loss rate, the sample consisted of at least 47 participants in each group.
Intervention program
The intervention program was comprised of two groups, G1 and G2, monitored throughout 90 days through four face-to-face meetings: Baseline (T0), 30 days after T0 (T1), 60 days after T0 (T2), and 90 days after T0 (T3). There were also weekly telephone reinforcements up to 60 days of follow-up, totaling four face-to-face meetings and six telephone contacts per group.
In T0 (baseline), the first face-to-face meeting took place with the participants from both groups (G1 and G2) to explain the objectives of the study and obtain the agreement to participate in the research. After accepting to participate in the study, the participants were led to a private room in the FHS. Afterwards, the instruments for sociodemographic and clinical characterization were applied, to evaluate the cognitive state, to measure the quality of sleep and the diabetes-related emotional distress. The participants Who obtained a total score of the PSQI > 5 points and sufficient cognitive state according to the level of schooling were randomized into both groups (G1 or G2) and were given the order to draw blood to measure A1c levels.
The participants from G1, during T0, received educational guidance on sleep hygiene based on national and international guidelines on DM and sleep (21, 22) in effect during the study period. Hygiene is considered a psycho-educational intervention, which consists of teaching the person to avoid external or environmental factors that generate adverse and harmful effects to sleep (22).
The orientation sessions lasted from 30 to 40 minutes and were conducted personally by the principal researcher through verbal orientations and leaflets. The orientations carried out involved aspects to promote improved quality of sleep, such as: Maintaining a quiet, dark environment and with pleasant temperature to sleep; going to bed only when sleepy; not reading, writing, eating, watching television, talking on the phone, or playing card games in bed; getting up in case of not falling asleep within 20 minutes; using relaxation techniques, like breathing, concentration, and stretching before going to sleep; maintaining regular hours for sleeping; not eating heavily prior to going to sleep, preferring small portions or a snack; not drinking coffee, black tea or mate, soft drinks, chocolate, or alcohol for four to six hours before bedtime; not smoking for at least four to six hours before bedtime; avoiding long rests after lunch (more than one hour) or decreasing daytime naps; avoiding physical activity four hours or less before bedtime; getting exposed to sunlight daily for 40 minutes in the first half of the morning or late afternoon. These periods were chosen as a protection measure against possible sun damage to the skin of the participants. During T1 and T2, the meetings were meant to reinforce the educational orientations and clear doubts expressed by the participants. This took place weekly via telephone contact between T0 and T1 and T1 and T2.
For G2, during T0, the participants received orientations about care of the feet, focused on hygiene, hydration, proper use of appropriate shoes and socks, and toenail cutting (21). Said orientation was proposed because it was a strategy already in use in the units where the data were collected and because it was not associated with the quality of sleep. This group also received face-to-face reinforcements and telephone contacts in the same way as G1, while reinforcing self-care guidelines with the feet. Lastly, during T3, the study variables of the participants from both groups (G1 and G2) were measured again. In this phase, a previously trained research collaborator collected the data.
The participants from groups G1 and G2 were composed of individuals from the four FHS units. To avoid contact among the participants from both groups, the schedules of the face-to-face meetings were carried out at intervals, so that the participants would not see each other in the health unit. During the data collection, the participants were also guided to not reveal the group to which they belonged.
Data collection
The sociodemographic and clinical variables were obtained through a semi-structured instrument and subdivided into sociodemographic information: Age in complete years, sex, marital status, schooling, occupation, monthly family income, and with whom they currently reside; clinical information: Time of DM2 treatment in months, co-morbidities, medications taken, performance of physical activity, smoking and consumption of alcoholic beverages were also variables addressed. Anthropometric measurements of weight, height, body mass index (BMI), and abdominal circumference were also performed.
To evaluate general cognitive functioning, the MMSE was used (19, 23). The instrument has 11 items with maximum score of 30 points adjusted according to the individual’s level of schooling: Illiterate: MMSE > 20 points; schooling of 1-4 years: MMSE > 25 points; schooling of 5-8 years: MMSE > 26.5 points; schooling of 9-11 years: MMSE > 28 points; schooling over 11 years: MMSE > 29 points (24).
The quality of sleep was measured through the Brazilian version of the PSQI (PSQUI-BR) (18). The internal consistency of this version measured through Cronbach’s alpha coefficient, was 0.73, which indicated high degree of internal consistency. This instrument evaluates the quality of sleep in relation to the last month and aims to provide a measure of standardized sleep quality between “good sleepers” and “bad sleepers”. It is composed of four open questions and six semi-open questions divided into seven domains: Subjective quality, latency, duration, habitual efficiency and sleep disorders, use of sleep medications and disorders during the day; the maximum score is 21 points. Scores above five points indicate poor quality of sleep pattern (18).
Diabetes-related emotional distress was measured through the Brazilian version of the Diabetes Distress Scale (B-DDS) (24). The instrument is composed of 17 items divided into four domains: Emotional burden (5 items); physician-related distress (4 items); regimen-related distress (5 items), and diabetes related interpersonal distress (3 items). The scale used is Likert type, which varies between 1 (it is not a problem) and 6 (it is a very important problem) points. The sum of the responses of the four domains divided by the number of items generates a total score that varies between 1 and 6. Values below 2.0 indicate little or no diabetes-related distress; values between 2.0 and 2.9 indicate moderate distress related to diabetes, and values ≥ 3.0 indicate high diabetes-related distress (25). All the data, except those of participant characterization, were collected during T0 and T3; the measurement of A1c was conducted within five days after T0. The method used to measure A1c followed the recommendations of the National Glyco-hemoglobin Standardization Program (NGSP) and was standardized by the reference of Diabetes Control and Complications Trial (DCCT) (26). Pre-intervention blood collection occurred in the laboratory of the municipality where the study took place, using the high-performance liquid chromatography technique.
Recruitment, randomization, allocation, and blinding
By means of a list of persons registered in the health units, the medical records of each of the eligible participants were accessed. Then the individuals were invited individually, by telephone, to participate in the study. On the scheduled day, the interviewer reinforced the invitation and explained the research steps. A member of the research team who was not in contact with the participants prepared a random sequence list generated by the website www.randomization.com, which considered the random-sized block method with 100 participants. The randomized list was sequentially numbered and sealed individually in opaque envelopes, to hide them from the researchers. A number exceeding the sample calculation was prepared due to possible losses during data collection. At the end of the first meeting (baseline), after implementing the measurement instruments, the principal researcher opened, in the sequence, each envelope and placed the participant in one of the groups (G1 or G2). At the end of the follow up, a research collaborator, who was not in contact with the study participants, collected the data to avoid collection bias.
Data analysis
Descriptive statistics was used to characterize the sample in the baseline, student t test, Mann-Whitney non-parametric test, chi-squared test. The Kolmogorov-Smirnov test was used to evaluate variable normality. For comparisons between groups with regard to the variables of sleep quality and emotional stress measured over time, linear models of mixed effects were proposed. For cases where the assumptions of the model were not met, the Box-Cox transformation was applied to the dependent variable.
A multiple linear regression model was constructed to evaluate the relationship between a set of independent variables (BMI, emotional stress, age, time of treatment, sex, marital status, people with whom they live, monthly family income, physical activity, and group) and quality of sleep. This model was adjusted by the quality of sleep at baseline. The value of the R2 explanation coefficient was reported for the adjusted model (27), which suggests the following classification of the R2 explanation coefficient of 0.01 up to 0.08 (weak); 0.09 to 0.24 (moderate); and ≥0.25 (strong). The analyses were performed through the SAS software version 9.4 and 5 % level of significance was considered.
Results
A total of 189 individuals had eligibility criteria. Of these, 87 did not meet the inclusion criteria, and two refused to participate in the study. There were losses throughout the follow up in both groups. Five participants did not continue in G1 and four in G2 because they started pharmacological treatment that interfered with sleep (Figure 1). None of the participants reported complications or any damage attributed to the intervention during the study program.
Figure 1. Flow diagram of the study participants per CONSORT (Paraguaçu, Minas Gerais, Brazil, 2016)
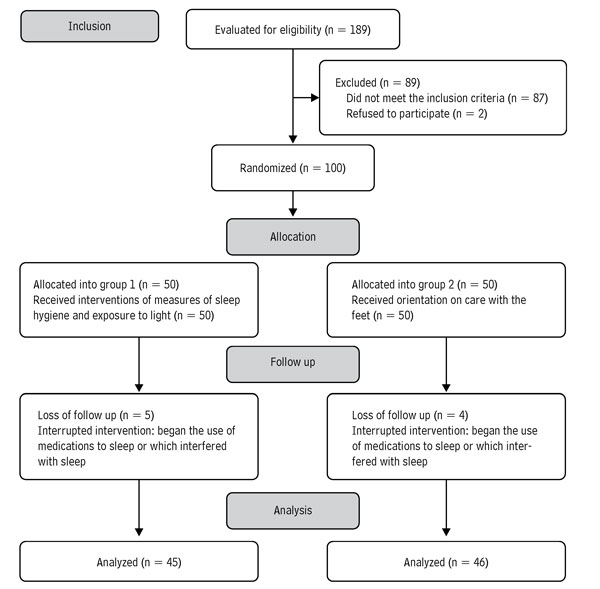
Source: Own elaboration.
Table 1 presents the sociodemographic and clinical characteristics of the participants at the baseline. The sample has predominance of women (57.14 %), with mean age of 54.87 ± 6.96 years. Most of the participants (71.43 %) had 1 to 8 years of schooling. The measurement of A1c was 8.18 ± 2.07. With the exception of gender and self-report of physical activity, there were no significant differences between the groups.
Table 1. Comparison of sociodemographic and clinical data among people with DM2 from G1 and G2 in the baseline (Paraguaçu, Minas Gerais, Brazil, 2016)
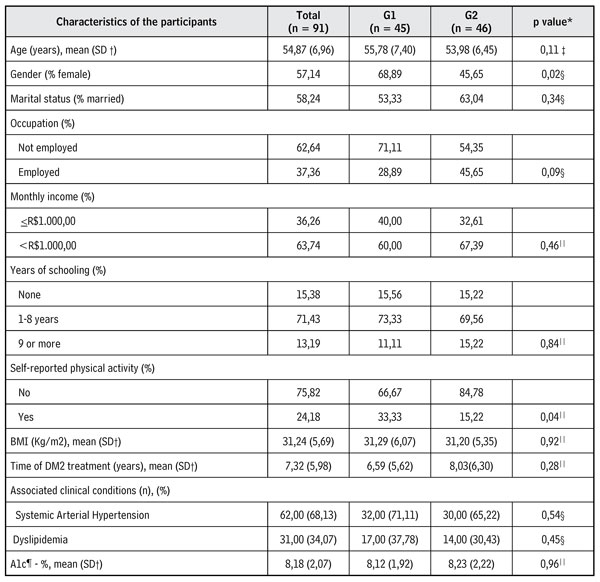
* p value, ? Standard
deviation, ‡ p
value obtained through the non-paired student t test, § p value obtained through the chi-squared test, || p value obtained through the Mann-Whitney test, ¶ Glycated hemoglobin.
Source: Own elaboration.
To compare the variables of quality of sleep and diabetes-related emotional distress (and their domains), linear models of mixed effects were used. In the patients included in G1, the results evidenced that the sleep hygiene strategies were effective in reducing the PSQI score (p < 0.01), however they were not able to characterize these participants as good sleepers (score of 5.02 at the end of follow-up). There was a significant decrease in the levels of diabetes-related emotional distress (p <0.01), in the domains related to physician-related distress (p <0.01) and regimen-related distress (p <0.01); thus, the sleep hygiene strategies contributed to reducing the levels of emotional stress from moderate (2.13 ± 1.10) to little or no emotional distress (1.51 ± 0.51). In addition, in G2, a significant reduction of PSQI levels was also observed (p <0.01), of diabetes-related emotional distress (p < 0.01), as well as of the domains of emotional burden (p < 0.01) and regimen-related distress (p < 0.01). A significantly higher PSQI score reduction was observed among G1 participants compared to G2 (p <0.02) at the end of follow-up (Table 2).
Table 2. Intra- and inter-group comparisons according to the variables over time — baseline and three months after follow up (Paraguaçu, Minas Gerais, Brazil, 2016)
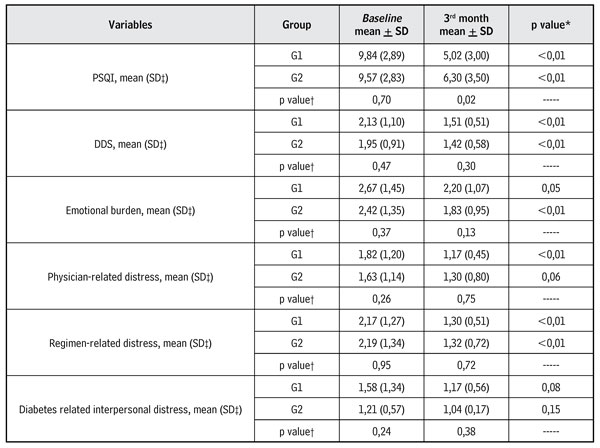
* intra-group p-value, †p-valor between groups, ‡ Standard deviation.
Source: Own elaboration.
Table 3 shows a multiple linear regression model constructed to evaluate the relationship between a set of independent variables and the dependent variable (quality of sleep). The quality of sleep at the end of the follow up was associated with the variables of diabetes-related emotional distress (p = 0.04), age (p = 0.04), sex (p = 0.01), and group (p < 0.01). The increase of 1 point in the DDS score contributed to the increase of 1.22 points in the PSQI score; in the age variable, the increase of one year in age implies an increase of 0.09 in the PSQI score; being of female gender meant an increase of 1.57 points in the PSQI score when compared to the individuals of male sex, and – finally – being included in G1 contributed to diminishing 1.77 points in the PSQI score when compared to G2. Thus, the model was able to explain 32 % of the variability of the dependent variable, with the correlation coefficient considered strong, according to Cohen (27) (Table 3).
Table 3. Multiple linear regression analysis between quality of sleep and the independent variables (Paraguaçu, Minas Gerais, Brazil, 2016)
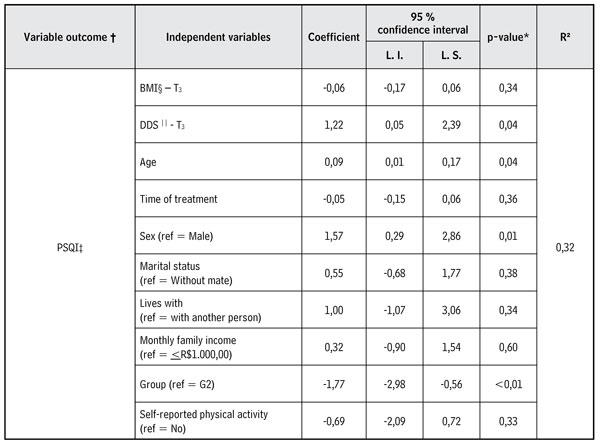
* p-value; ? the model was adjusted by the PSQI measurement in T0, ‡ Pittsburgh
sleep quality index, § Body Mass Index, || Diabetes Distress Scale.
Source: Own elaboration.
Discussion
This study evaluated educational strategies to improve the quality of sleep and the relationship with emotional stress associated with diabetes. The reduced mean values of the PSQI and DDS scores were statistically significant and describe improved quality of sleep and of the levels of emotional stress in the participants from G1, which is corroborated by previous studies (28, 29).
A randomized clinical trial investigating the effects of a non-pharmacological care plan, which included sleep hygiene and self-care guidelines in people with DM2, revealed similar results to our study, with significant reduction in the score of quality of sleep (PSQI from 9.05 to 4.79) in the group receiving said care plan after 75 days of follow-up; however, no significant differences were found between the groups studied (29). At the same time, another study using sleep hygiene measures in hemodialysis patients also observed a statistically significant difference between the groups at the end of the sleep quality follow-up with a reduction in the mean PSQI score from 12.7 to 7.03 (30).
In this study, regarding diabetes-related emotional distress, the participants included in G1 showed significant reduction throughout the follow up. Studies indicate the existence of an association between sleep and diabetes-related distress, given that reduced sleep time refers to an increase of this stress, and these factors can impact upon the quality of life of individuals with DM2 (31-33). As guidelines are developed to improve sleep, it is also possible to observe reduced levels of diabetes-related emotional distress.
The variables of belonging to G1, age, and female sex were also involved in the quality of sleep. Being included in G1 contributed to a significant reduction of 1.86 points in the PSQI score when compared with G2. In turn, being female has significantly contributed to worsening sleep quality. These results confirm what has already been described previously, that is, the strong relation between low quality of sleep and being female (34, 35). Age was another variable that also contributed to worsening the quality of sleep, given that the increase of one year contributed to a worse PSQI score, in a mean of 0.09. This result agrees with a study carried out in Brazil with a sample of 1,024 participants, between 20 and 80 years of age in which a linear increase was observed between waking after the onset of sleep and aging. In addition, total sleep time, sleep efficiency, and slow-wave sleep were also reduced with aging, which indicated a negative factor with aging. It is highlighted that these results were not related with gender (36).
Another finding of the study, although unexpected, was the fact that G2 showed improvement in sleep quality scores. However, this reduction was not sufficient to classify patients as “good sleepers”. There were also reduced levels of diabetes-related emotional distress. Moreover, this group was initially classified with little or no diabetes-related distress. The fact that both groups received orientations focused on improving self-care, whether sleep hygiene guidelines or foot care, may have influenced this finding. Another hypothesis would be contact between participants from G1 and G2, which may have contributed to the exchange of knowledge of actions directed toward sleep hygiene. Thus, in the future and as a continuation of this study, it would be important to investigate the effectiveness of these interventions together with a “pure” control group, that is, based on the usual treatment received in the service, field of study.
The strong points of this study were to involve educational strategies associated with telephone reinforcement, in addition to performing the study in the primary care service, where most people with DM2 are followed up, which allows replicating the strategies of this study in other services with the same level of complexity. However, this study has some limitations: The absence of a formal recording instrument (sleep log) for the participants, measurement of the quality of sleep by subjective instrument, and failure to measure how much and how the participants adhered to the sun exposure; in addition, the absence of control over the fact that the day is overcast or rainy, as well as the seasons of the year, the short follow-up period of the participants, and the possibility of contaminating the control group (because they are units with low number of users; exchange of knowledge among participants, contact with information provided by the media or even other health professionals) are also possible limitations.
Conclusion
After 90 days of follow up it is concluded with this study that conducting face-to-face educational strategies based on sleep hygiene (with verbal orientation and leaflets), combined with telephone reinforcements, were effective to improve sleep quality in patients with DM2, measured through the PSQI-BR instrument, and of the diabetes-related emotional distress, evaluated through the B-DDS instrument. Said educational orientations are low-cost strategies and can be implemented in the nurse’s clinical practice together with patients with DM2.
Conflict of interest: None declared.
References
1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. DOI: 10.1016/j.diabres.2017.03.024
2. Zeng Y, Wu J, Yin J, Chen J, Yang S, Fang Y. Association of the combination of sleep duration and sleep quality with quality of life in type 2 diabetes patients. Qual Life Res. 2018; 27(12):3123-30. DOI: 10.1007/s11136-018-1942-0
3. McNeil J, Doucet É, Chaput JP. Inadequate sleep as a contributor to obesity and type 2 diabetes. Can J. Diabetes. 2013;37(2):103-8. DOI: 10.1016/j.jcjd.2013.02.060
4. Barone MT, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91(2):129-37. DOI: 10.1016/j.diabres.2010.07.011
5. Gupta S, Wang Z. Predictors of sleep disorders among patients with type 2 diabetes mellitus. Diabetes Metab Syndr. 2016;10(4):213-20. DOI: 10.1016/j.dsx.2016.06.009
6. Ryan S. Sleep and diabetes. Curr Opin Pulm Med. 2018;24(6):555-60. DOI: 10.1097/MCP.0000000000000524
7. Sakamoto R, Yamakawa T, Takahashi K, Suzuki J, Shinoda MM, Sakamaki K et al. Association of usual sleep quality and glycemic control in type 2 diabetes in Japanese: A cross sectional study. Sleep and Food Registry in Kanagawa (SOREKA). PLoS One. 2018;13(1):e0191771. DOI: 10.1371/journal.pone.0191771
8. Khandelwal D, Dutta D, Chittawar S, Kalra S. Sleep Disorders in Type 2 Diabetes. Indian J Endocrinol Metab. 2017;21(5):758-61. DOI: 10.4103/ijem.IJEM_156_17
9. Luyster FS, Dunbar-Jacob J. Sleep quality and quality of life in adults with type 2 diabetes. Diabetes Educ. 2011;37(3):347-55. DOI: 10.1177/0145721711400663
10. Stanković Z, Jasović-Gasić M, Lecić-Tosevski D. Psychological problems in patients with type 2 diabetes — clinical considerations. Vojnosanit Pregl. 2013;70(12):1138-44.
11. Ogbera A, Adeyemi-Doro A. Emotional distress is associated with poor self-care in type 2 diabetes mellitus. J Diabetes. 2011;3(4):348-52. DOI: 10.1111/j.1753-0407.2011.00156.x
12. Bloom HG, Ahmed I, Alessi CA, Ancoli-Israel S, Buysse DJ, Kryger MH et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57(5):761-89. DOI: 10.1111/j.1532-5415.2009.02549.x
13. Wu MC, Sung HC, Lee WL, Smith GD. The effects of light therapy on depression and sleep disruption in older adults in a long-term care facility. Int J Nurs Pract. 2015;21(5):653-9. DOI: 10.1111/ijn.12307
14. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. DOI: 10.1016/j.smrv.2016.02.001
15. Düzgün G, Durmaz Akyol A. Effect of Natural Sunlight on Sleep Problems and Sleep Quality of the Elderly Staying in the Nursing Home. Holist Nurs Pract. 2017;31(5):295-302. DOI: 10.1097/HNP.0000000000000206
16. Boubekri M, Cheung IN, Reid KJ, Wang CH, Zee PC. Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. J Clin Sleep Med. 2014;10(6):603-11. DOI: 10.5664/jcsm.3780
17. Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726-32. DOI: 10.7326/0003-4819-152-11-201006010-00232
18. Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, Miozzo IC, de Barba ME et al. Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011;12(1):70-5. DOI: 10.1016/j.sleep.2010.04.020
19. Bertolucci PH, Brucki SM, Campacci SR, Juliano Y. The Mini-Mental State Examination in a general population: impact of educational status. Arq Neuropsiquiatr. 1994;52(1):1-7. DOI: 10.1590/S0004-282X1994000100001
20. Saeedi M, Shamsikhani S, Varvani Farahani P, Haghverdi F. Sleep hygiene training program for patients on hemodialysis. Iran J Kidney Dis. 2014;8(1):65-9.
21. Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM, Association AD. Preventive foot care in people with diabetes. Diabetes Care. 2003;26(suppl 1):S78-9. DOI: 10.2337/diacare.26.2007.s78
22. Brazilian Sleep Association, Bacelar A, Pinto Júnior LR, editors. Insônia: do diagnóstico ao tratamento — III Consensus Brazilian Insomnia. São Paulo: Omnifarma; 2013. [cited 2018 oct 09]; Available from: http://www.absono.com.br/consenso-brasileiro-de-insonia.html
23. Brucki SM, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH. Suggestions for utilization of the mini-mental state examination in Brazil. Arq Neuropsiquiatr. 2003;61(3B):777-81. DOI: 10.1590/S0004-282X2003000500014
24. Apolinario PP, Trevisan DD, Rodrigues RC, Jannuzzi FF, Ferreira JF, de Oliveira HC, et al. Psychometric Performance of the Brazilian Version of the Diabetes Distress Scale in Patients With Diabetes Mellitus Type 2. J Nurs Meas. 2016;24(2):101-13. DOI: https://doi.org/10.1891/1061-3749.24.2.E101
25. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-64. DOI: 10.2337/dc11-1572
26. Association AD. (6) Glycemic targets. Diabetes Care. 2015;38(suppl):S33-40. DOI: 10.2337/dc15-S009
27. Cohen J. A power primer. Psychol Bull. 1992;112(1):155-9.
28. Keskin A, Ünalacak M, Bilge U, Yildiz P, Güler S, Selçuk EB et al. Effects of Sleep Disorders on Hemoglobin A1c Levels in Type 2 Diabetic Patients. Chin Med J (Engl). 2015;128(24):3292-7. DOI: 10.4103/0366-6999.171415
29. Khosravan S, Alami A, Golchin Rahni S. Effects of continuous care model based non-pharmacological intervention on sleep quality in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Community Based Nurs Midwifery. 2015;3(2):96-104.
30. Soleimani F, Motaarefi H, Hasanpour-Dehkordi A. Effect of Sleep Hygiene Education on Sleep Quality in Hemodialysis Patients. J Clin Diagn Res. 2016;10(12):LC01-4. DOI: 10.7860/JCDR/2016/19668.8941
31. Seligowski AV, Pless Kaiser AP, Niles BL, Mori DL, King LA, King DW. Sleep quality as a potential mediator between psychological distress and diabetes quality of life in veterans with type 2 diabetes. J Clin Psychol. 2013;69(10):1121-31. DOI: 10.1002/jclp.21866
32. Reutrakul S, Siwasaranond N, Nimitphong H, Saetung S, Chirakalwasan N, Ongphiphadhanakul B et al. Relationships among sleep timing, sleep duration and glycemic control in Type 2 diabetes in Thailand. Chronobiol Int. 2015;32(10):1469-76. DOI: 10.3109/07420528.2015.1105812
33. Zhou H, Zhu J, Liu L, Li F, Fish AF, Chen T et al. Diabetes-related distress and its associated factors among patients with type 2 diabetes mellitus in China. Psychiatry Res. 2017;252:45-50. DOI: 10.1016/j.psychres.2017.02.049
34. Andrechuk CR, Ceolim MF. Sleep quality and adverse outcomes for patients with acute myocardial infarction. J Clin Nurs. 2016;25(1-2):223-30. DOI: 10.1111/jocn.13051
35. Hung HC, Yang YC, Ou HY, Wu JS, Lu FH, Chang CJ. The association between self-reported sleep quality and overweight in a Chinese population. Obesity (Silver Spring). 2013;21(3):486-92. DOI: 10.1002/oby.20259
36. Moraes W, Piovezan R, Poyares D, Bittencourt LR, Santos-Silva R, Tufik S. Effects of aging on sleep structure throughout adulthood: a population-based study. Sleep Med. 2014;15(4):401-9. DOI: 10.1016/j.sleep.2013.11.791